-
Inhibition of XPO-1 Mediated Nuclear Export through the Michael-Acceptor Character of Chalcones
Gargantilla, M.; López-Fernández, J.; Camarasa, M. J.; Persoons, L.; Daelemans, D.; Priego, E. M.; Pérez-Pérez, M. J.
Pharmaceuticals 2021, 14, 1311
-
Tlr4 antagonism reduces movement-induced nociception and atf-3 expression in experimental osteoarthritis
Ferreira-Gomes, J.; García, M. M.; Nascimiento, D.; Almeida, L.; Quesada, E.; Castro-Lopes, J. M.; Pascual, D.; Goicoechea, C.; Neto, F. L.
Journal of Pain Research 2021, 14, 2615-2627
-
Structural insights into the mechanisms of action of functionally distinct classes of Chikungunya virus nonstructural protein 1 inhibitors
Kovacikova, K.; González, M. G.; Jones, R.; Reguera, J.; Gigante, A.; Pérez-Pérez, M. J.; Pürstinger, G.; Moesslacher, J.; Langer, T.; Jeong, L. S.; Delang, L.; Neyts, J.; Snijder, E. J.; van Westen, G. J. P.; van Hermet, M. J.
Antimicrobial Egents and Chemotherapy 2021, 65, e02566-20
-
Small Molecule–Peptide Conjugates as Dimerization Inhibitors of Leishmania infantum Trypanothione Disulfide Reductase
Revuelto, A.; López-Martín, I.; de Lucio, H.; García-Soriano, J. C.; Zanda, N.; de Castro, S.; Gago, F.; Jiménez-Ruiz, A.; Velázquez, S.; Camarasa, M. J.
Pharmaceuticals 2021, 14, 689
-
Double Arylation of the Indole Side Chain of Tri- and Tetrapodal Tryptophan Derivatives Renders Highly Potent HIV-1 and EV71 Entry Inhibitors
Martí-Marí, O.; Martínez-Gualda, B.; de la Puente-Secades, S.; Mills, A.; Quesada, E.; Abdelnabi, R.; Sun, L.; Boonen, A.; Noppen, S.; Neyts, J.; Schols, D.; Camarasa, M. J.; Gago, F.; San-Félix, A.
Journal of Medicinal Chemistry 2021, 64, 10027-10046
-
Multivalent Tryptophan- and Tyrosine-Containing [60]Fullerene Hexa-Adducts as Dual HIV and Ev71 Entry Inhibitors
Ruiz-Santaquiteria, M.; Illescas, B. M.; Abdelnabi, R.; Boonen, A.; Mills, A.; Martí-Marí, O.; Noppen, S.; Neyts, J.; Schols, D.; Gago, F.; San-Félix, A.; Camarasa, M. J.; Martín, N.
Chemistry, A European Journal 2021, 27, 10700-10710
-
Highly Efficient Dimerization Disruption of Leishmania infantum Trypanothione Reductase by Triazole-phenylthiazoles.
Revuelto, A. De Lucio, J.C. García-Soriano, P. Sánchez-Murcia, F. Gago, A. Jiménez-Ruiz, M.J. Camarasa, S. Velázquez.
Journal of Medicinal Chemistry 2021, 64, 6137-6160
-
HIV-1 Envelope Spike MPER: From a Vaccine Target to a New Druggable Pocket for Novel and Effective Fusion Inhibitors.
Luke, F. J.; Camarasa, M. J.
ChemMedChem 2021, 16, 105-107
-
Pseudoirreversible slow-binding inhibition of trypanothione reductase by a protein-protein interaction disruptor.
de Lucio, H.; Toro, M. A.; Camarasa, M. J.; Velázquez, S.; Gago, F.; Jiménez-Ruiz, A.
Brithish Journal of Pharmacology 2020, 177, 5163
-
Peptides mimicking the b7/b8 loop of HIV-1 Reverse Transcriptase p51 as “Hotspot-Targeted” Dimerization Inhibitors.
Sánchez-Murcia, P. A.; de Castro, S.; García-Aparicio, C.; Jiménez, M. A.; Corona, A.; Tramontano, E.; Sluis-Cremer, N.; Menéndez-Arias, L.; Velázquez, S.; Gago, F.; Camarasa, M. J.
ACS Medicinal Chemistry Letters 2020, 11, 811-817
-
N-benzyl 4,4-disubstituted piperidines as a potent class of influenza H1N1 virus inhibitors showing a novel mechanism of hemagglutinin fusion peptide interaction.
de Castro, S.; Ginex, T.; Vanderlinden, E.; Laporte, M.; Stevaert, A.; Cumella, J.; Gago, F.; Camarasa, M. J.; Luque, F. J.; Naesens, L.; Velázquez, S.
European Journal of Medicinal Chemistry 2020, 194, 112223.
-
Tryptophan trimers and tetramers inhibit dengue and Zika virus replication by interfering with viral attachment processes.
Fikatas, A.; Vervaeke, P.; Martínez-Gualda, B.; Martí-Marí, O.; Noppen, S.; Meyen, E.; Camarasa, M. J.; San-Félix, A.; Pannecouque, C.; Schols. D.
Antimicrobial Agents and Chemotherapy 2020, 64(3), e02130-19
-
Scaffold simplification strategy leads to a novel generation of dual HIV and enterovirus-A71 entry inhibitors.
Martínez-Gualda, B.; Sun, L.; Martí-Marí, O.; Noppen, S.; Abdelnabi, R.; Bator, C. M.; Quesada, E.; Delang, L.; Mirabelli, C.; Lee, H.; Schols, D.; Neyts, J.; Hafenstein, S.; Camarasa, M. J.; Gago, F.; San-Félix, A.
Journal of Medicinal Chemistry 2020, 63, 349-368.
-
Design and synthesis of new 6-nitro and 6-amino-3,3,4,4-tetrahydro-2H-benzo[g]indazole derivatives: antiproliferative and antibacterial activity.
Cuartas,V.; Crespo, M. P.; Priego, E. M.; Persoons, L.; Daelemans, D.; Camarasa, M J.; Insuasty, B.; Pérez-Pérez, M. J.
Molecules 2019, 24, 436
Enlace a digital.csic
-
Chikungunya virus drug discovery: still a long way to go?.
Pérez-Pérez, M. J.; Delang, L.; Ng, L. F. P.; Priego, E. M.
Expert Opinion in Drug Discovery 2019, 14, 855-866
Enlace a digital.csic
-
Chalcones and Chalcone-mimetic Derivatives as Notch Inhibitors in a Model of T‑cell Acute Lymphoblastic Leukemia
.
Quaglio, D.; Zhdanovskaya, N.; Tobajas, G.; Cuartas, V.; Balducci, S.; Christodoulou, M. S.; Fabrizi, G.; Gargantilla, M.; Priego, E. M.; Carmona Pestaña, A.; Passarella, D.; Screpanti, I.; Botta, B.; Palermo, R.; Mori, M.; Ghirga, F.; Pérez-Pérez, M. J.
ACS Medicinal Chemistry Letters 2019, 10, 639-643
Enlace a digital.csic
-
Approved drugs screening against the nsP1 capping enzyme of Venezuelan equine encephalitis virus using an immuno-based assay.
Ferreira-Ramos, A. S.; Li, C. Q.; Eydoux, C.; Contreras, J. M.; Morice, C.; Querat, G.; Gigante, A.; Pérez-Pérez, M. J.; Jung, M. L.; Canard, B.; Guillemot, J. C.; Decroly, E.; Coutard, B.
Antiviral Research 2019, 163, 50-69
Enlace a digital.csic
-
(F)uridylylated Peptides Linked to VPg1 of Foot-and- Mouth Disease Virus (FMDV): Design, Synthesis and X-Ray Crystallography of the Complexes with FMDV RNA-dependent RNA polymerase.
De Castro, S.; Ferrer-Orta, C.; Mills, A.; Fernández-Cureses, G.; Gago, F.; Verdaguer, N.; Camarasa, M.J.
Molecules 2019, 24, 2360-2374
-
Modifications in the branched arms of a class of dual inhibitors of HIV and EV71 replication expand their antiviral spectrum.
Martínez-Gualda, B.; Sun, L.; Martí-Marí, O.; Mirabelli, C.; Delang, L.; Neyts, J.; Schols, D.; Camarasa, M. J.; San-Félix, A.
Antiviral Research 2019, 168, 210-214
-
Viral engagement with host (co-)receptors blocked by a novel class of tryptophan dendrimers that targets the 5-fold-axis of the enterovirus-A71 capsid.
Sun, L.; Lee, H.; Thibaut, H.J.; Rivero-Buceta, E.; Martínez-Gualda, B.; Delang, L.; Leyssen, P.; Gago, F.; San-Félix, A.; Hafenstein, S.; Mirabelli, C.; Neyts, J.
Plos Pathogens 2019, 15, e1007760
-
From peptide to non-peptide dimerization inhibitors of trypanothione reductase with potent activity against Leishmania infantum: the pyrrolopyrimidine vs the imidazole-phenyl-thiazole scaffold.
Revuelto, A.; Ruiz-Santaquiteria, M.; de Lucio, H.; Gamo, A.; Carriles, A. A.; Gutiérrez, K. J.; Sánchez-Murcia, P. A.; Hermoso, J. A.; Gago, F.; Camarasa, M. J.; Jiménez-Ruiz, A.; Velázquez, S.
ACS Infect. Dis. 2019, 5, 873-891
-
Galloyl carbohydrates with antiangiogenic activity mediated by Capillary Morphogenesis Gene 2 (CMG2) protein binding.
Doyagüez, E.; Carrero, P.; Madrona, A.; Martínez-Gualda, B.; Camarasa, M.-J.; Jimeno, M. L.; Bennallack, P.; Finnell, J. ; Tsang, T.; Christensen, K.; San-Felix, A.; Rogers, M.
J. Med. Chem. 2019, 62, 3958-3979
-
Diphenyl ether derivatives occupy the expanded binding site of cyclohexanedione compounds at the colchicine site in tubulin by movement of the αT5 loop.
Bueno, O.; Gargantilla, M.;Estévez-Gallego, J. ; Martins, S.; Díaz, J. F.; Camarasa, M. J.; Liekens, S.; Pérez-Pérez, M. J.; Priego, E. M.
Eur. J. Med. Chem. 2019, 148, 337-348.
Enlace a digital.csic
-
Enzymatic and Chemical synthesis of nucleic acid derivatives
Fernández-Lucas, J.; Camarasa, M. J. (Editors)
Willey, ISBN:978-3-527-81209-7
-
Conformational mimetics of the α-methyl chalcone TUB091 binding tubulin: Design, synthesis and antiproliferative activity.
Bueno,O. ; Tobajas, G.; Quesada, E.; Estévez-Gallego, J.; Camarasa, M. J.; Díaz, J. F.; Liekens, S.; Priego, E. M.; Pérez-Pérez, M. J.
Eur. J. Med. Chem. 2018, 148, 337-348.
Enlace a digital.csic -
Trypanothione reductase inhibition and anti-leishmanial activity of all-hydrocarbon stapled -helical peptides with improved proteolytic stability.
Ruiz-Santaquiteria, M.; De Castro, S.; Toro, M. A.; De Lucio, H.; Guitérrez, K. J.;. Sánchez-Murcia, P. A; Jiménez, M. A.; Gago, F.; Jiménez-Ruiz, A.; Camarasa, M. J.; Velázquez, S.
Eur. J. Med. Chem. 2018 149, 238-247.
-
Polypharmacology in HIV inhibition: can a drug with simultaneous action over two relevant targets be an alternative to combination therapy?.
De Castro, S.;Camarasa, M. J.
Eur. J. Med. Chem. 2018 150, 206-227.
-
High-affinity ligands of the colchicine domain in tubulin based on a structure-guided design.
Bueno,O. ; Estévez-Gallego, J.; Martins, S.; Prota, A. E.; Gago, F.; Gómez-SanJuan, A.; Camarasa, M. J.; Barasoain, I.; Steinmetz, M. O.; Díaz, J. F.; Pérez-Pérez, M. J.; Liekens, S.; Priego, E. M.
Scientific. Reports 2018, 8, 4242-4258.
-
Inhibition of the replication of different strains of chikungunya virus by 3-aryl-[1,2,3]triazolo [4,5-d]pyrimidin-7(6H)-ones.
Gómez-SanJuan, A.; Gamo, A. M.; Delang, L.; Pérez, A.; Amrun, S. N.;Abdelnabi, R.; Jacobs, S.; Priego, E. M.; Camarasa, M. J.; Jochmans, D.; Leyssen, P.;Ng, L. F. P.; Querat, G.; Neyts, J.; Pérez-Pérez, M. J.
ACS Infect. Dis. 2018, 13, 605-619
-
A novel class of cationic and non-peptidic small molecules as hits for the development of antimicrobial agents.
Jiménez, A.; García, P.; de la Puente, S.; Madrona, A.; Camarasa, M. J.; Pérez-Pérez, M. J.; Quintela, J. C.; García-del Portillo, F.; San-Félix, A.
Molecules 2018, 23, 1513.
-
Prodrugs of nucleoside triphosphates a sound and challenging approach. A pioneering work that opens a new era in the direct intracellular delivery of nucleoside triphosphates
Camarasa, M. J.
ChemMedChem 2018, 13, 1885-1889.
-
Novel Polyphenols That Inhibit Colon Cancer Cell Growth Affecting Cancer Cell Metabolism.
Gómez de Cedrón, M.; Vargas, T.; Madrona, A.; Jiménez, Aranza; Pérez-Pérez, M.-J.; Quintela, J.-C.; Reglero, G.; San-Félix, A.; Ramírez de Molina, A.
J. Pharmacol. Exp. Ther. 2018, 366, 377-389.
-
Antivascular and antitumor properties of the tubulin-binding chalcone TUB091.
Canela, M. D.; Noppen, S.; Bueno, O.; Prota, A. E.; Bargsten, K.; Sáez-Calvo, G.; Jimeno, M. L.; Benkheil, M.; Ribatti, D.; Velázquez, S.; Camarasa, M. J.; Díaz, J. F.; Steinmetz, M. O.; Priego, E. M.; Pérez-Pérez, M. J.; Liekens, S.
Oncotarget 2017, 8, 14325-14342
-
Structure-activity relationship studies on a Trp dendrimer with dual activities against HIV and enterovirus A71. Modifications on the amino acid.
Martínez-Gualda, B.; Sun, L.; Rivero-Buceta, E.; Quesada, E. ;Balzarini, J.; Noppen, S.; Liekens, S.; Schols, D.; Neyts, J.; Leyssen, P.; Mirabelli, C.; Camarasa, M.J.; San-Félix, A.
Antiviral Res. 2017 139, 32-40
-
First example of peptides targeting the dimer interface of Leishmania infantum trypanothione reductase with potent in vitro antileishmanial activity.
Ruiz-Santaquiteria, M.; Sánchez-Murcia, P. A.; Toro, M. A.; de Lucio, H.; Gutierrez, K.; De Castro, S.; Carneiro, F. A. C.; Gago, F.; Jiménez-Ruiz, A.; Camarasa, M.-J.; Velázquez, S.
Eur. J. Med. Chem. 2017, 135, 49-59.
-
Antiviral Activity of [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones against chikungunya virus targeting the viral capping nsP1./
Gigante, A.; Gómez-SanJuan, A.; Delang, L.; Li, C.; Bueno, O.; Gamo, A. M.; Priego, E. M.; Camarasa, M. J.; Jochmans, D.; Leyssen, P.; Decroly, E.; Coutard, B.;Querat, G.; Neyts, J.; Pérez-Pérez, M. J.
Antiviral Res. 2017, 144, 216-222.
-
Improved proteolytic stability and potent activity against Leishmania infantum trypanothione reductase of /B-peptide foldamers conjugated to cell-penetrating peptides.D
De Lucio, H.; Gamo, A.; Ruiz-Santaquiteria, M.; De Castro, S.; Sánchez-Murcia, P. A.; Toro, M. A.; Gutierrez, K. J.; Gago, F.; Jiménez-Ruiz, A.; Camarasa, M.J.; Velázquez S.
Eur. J. Med. Chem. 2017, 140, 615-623.
-
The viral capping enzyme nsP1: a novel target for the inhibition of chikungunya virus infection
Delang, L.; Li, C.; Tas, A.; Querat, G.; Albulescu, I. C.; De Burghgraeve, T.; Guerrero, N. A. S.; Gigante, A.; Piorkowoski, G.; Antiviral Res. 2016 139, 32-40
-
Optimization of a class of tryptophan dendrimers that inhibit HIV replication leads to a selective, specific and low-nanomolar inhibitor of clinical isolates of enterovirus A71
Rivero-Buceta, E.; Sun, L.; Martínez-Gualda, B.; Doyagüez, E.G.; Donckers, K.; Quesada, E.; Camarasa, M.J.; Delang, L.; San-Félix, A.; Neyts J.; Leyssen, P.
Antimicrob. Agents Chemother. 2016, 60,5064-5067
-
Blocking blood flow to solid tumors by destabilizing tubulin: an approach to targeting tumor growth
Pérez-Pérez, M. J.; Priego, E. M.; Bueno, O.; Martins, M. S.; Canela, M. D.; Liekens, S.
J. Med. Chem. 2016, 59, 8685-8711
-
Targeting the colchicine site in tubulin through cyclohexanedione derivatives
Canela, M. D.; Bueno, O.; Noppen, S.; Sáez-Calvo, G.; Estévez Gallego, J.; Díaz, J. F.; Camarasa, M. J.; Liekens, S.; Pérez-Pérez, M. J.; Priego, E. M.
RSC Adv. 2016, 6, 19492-19506.
-
2015- Rivero-Buceta, E.; Carrero, P.; Casanova, E.; Doyagüez, E. G.; Madrona, A.; Quesada, E.; Peréz-Pérez, M. J.; Mateos, R., Bravo, L.; Mathys, L.; Noppen, S.; Kiselev, E.; Marchand, C.; Pommier, Y.; Liekens, S.; Balzarini, J.; Camarasa, M. J.; San-Félix, A.
Anti-HIV-1 of a tripodal receptor that recognizes mannose oligomers. Eur. J. Med. Chem. 2015, 106, 132-143.
-
2015-Rivero-Buceta, E.; Carrero, P.; Doyagüez, E. G.; Madrona, A.; Quesada, E.; Camarasa, M .J.; Pérez-Pérez, M. J.; Leyssen, P.; Paeshuyse, J.; Balzarini, J.; Neyts, J.; San-Félix, A.
Linear and branched alkyl-esters and amides of gallic acid and other (mono-, di- and tri-) hydroxy benzoyl derivatives as promising anti-HCV inhibitors. Eur. J. Med. Chem. 2015, 92, 656-671.
-
2015-Fernández-Cureses, G.; De Castro, S.; Jimeno, M. L.; Balzarini, J.; Camarasa, M. J.
Design, synthesis and biological evaluation of a novel class of unconventional aminopyrimidine, aminopurine and amino-1,3,5-triazine methyloxy-nucleosides. ChemMedChem 2015, 10, 321-335.
-
2015-Velázquez, S.; De Castro, S.; Diez-Torrubia, A.; Balzarini, J.; Camarasa, M. J.
Dipeptidyl-peptidase IV (DPPIV/CD26)-activated prodrugs: a succesful strategy for improving wáter solubility and oral bioavailability. Curr. Med. Chem. 2015, 22, 1041-1054.
-
2015- Sánchez-Murcia, P. A.; Ruiz-Santaquiteria, M.; Toro, M. A.; de Lucio, H.; Jiménez, M. A.; Gago, F.; Jiménez-Ruiz, A.; Camarasa, M.-J.; Velázquez, S.
Comparison of hydrocarbon- and lactambridged cyclic peptides as dimerization inhibitors of Leishmania infantum trypanothione reductase. RSC Advances 2015,5, 55784-55794.
-
2015- de Castro, S.; Fernández-Cureses, G.; Andrei, G.; Sánchez-Murcia, P. A.; Korba, B.; Gago, F.; Balzarini, J.; Camarasa, M. J.
Conservation of antiviral activity and improved selectivity in PMEO-DAPym upon pyrimidine to triazine scaffold hopping. Antiviral Res. 2015,122, 64-68.
-
2015- Rivero-Buceta, E.; Doyagüez, E. G.; Colomer, I.; Quesada, E.; Mathys, L.; Noppen, S.; Liekens, S.; Camarasa, M. J.; Peréz-Pérez, M. J.; Balzarini, J.; San-Félix, A.
Tryptophan dendrimers that inhibit HIV replication, prevent virus entry and bind to the HIV envelope glycoproteins GP120 and GP41. Eur. J. Med. Chem. 2015,106, 34-43.
-
2014- Flores, A.; Camarasa, M. J.; Pére-Pérez, M. J.; San-Félix, A.; Balzarini, J.; Quesada, E.
Multivalent agents containing 1-substituted 2,3,4-trihydroxyphenyl moieties as novel synthetic polyphenols directed against HIV-1. Org. Biomol. Chem. 2014, 12, 5278-5294.
-
2014- Canela, M. D.; Liekens, S.; Camarasa, M. J.; Priego, E. M.; Pérez-Pérez, M. J.
Synthesis and antiproliferative activity of 6-phenylaminopurines. Eur. J. Med. Chem., 2014, 87, 421-428
-
2014- Gigante, A.; Canela, M. D.; Delang, L.; Priego, E. M.; Camarasa, M. J.; Querat, G.; Neyts, J.; Leyssen, P.; Pére-Pérez, M. J.
Identification of [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones as novel inhibitors of Chikunyunga Virus replication. J. Med. Chem, 2014, 57, 4000-4008
-
2014- Canela, M. D.; Pérez-Pérez, M. J.; Noppen, S.; Sáez-Calvo, G.; Díaz, J. F.; Camarasa, M.-J.; Liekens, S.; Priego, E.-M.
Novel Colchicine-Site Binders with a Cyclohexanedione Scaffold Identified through a Ligand-Based Virtual Screening Approach. J. Med. Chem, 2014, 57, 3924-3938
-
2014- de Castro, S.; Camarasa, M. J.; Balzarini, J.; Velázquez, S.
Discovery and SAR studies of a novel class of cytotoxic 1,4-disubstituted piperidines via Ugi reaction. Eur. J. Med. Chem., 2014, 83, 174-189.
-
2014- Gigante, A.; Priego E. M.; Sánchez-Carrasco, P.; Ruiz-Pérez, L. M.; Vande Voorde, J.; Camarasa, M. J.; Balzarini, J.; González-Pacanowska, D.; Pérez-Pérez, M. J.
Microwave-assisted synthesis of C-8 aryl and heteroaryl inosines and determination of their inhibitory activities against Plasmodium falciparum purine nucleoside phosphorylase. Eur. J. Med. Chem. 2014, 82, 459-465
-
2014- Thibaut, H. J.; van der Linden, L.; Jiang, P.; Thys, B.; Canela, M. D.; Aguado, L.; Rombaut, B.; Wimmer, E.; Paul, A.; Pérez-Pérez, M. J. van Kuppeveld, F. J. M.; Neyts, J.
Binding of glutathione to Enterovirus capsids is essential for virion morphogeneis. PLOS Pathogens, 2014, 10(4), e1004039.
-
2014 - Rico, E.; Oliva Correia, C.; Alzate, J. F.; Genes, C. M.; Moreno, D.; Casanova, E.; Pérez-Pérez, M. J.; Camarasa, M. J.; Clos, J.; Gago, F.; Jiménez-Ruiz, A.
Leishmanina infantum EndoG is an endo/exo-nuclease essential for parasite survival. PLOS ONE, 9 (2), e89526
-
2014 - Camarasa, M. J.
Heterocyclic Chemistry in Drug Discovery. ChemMedChem 2014, 9, 233-235
-
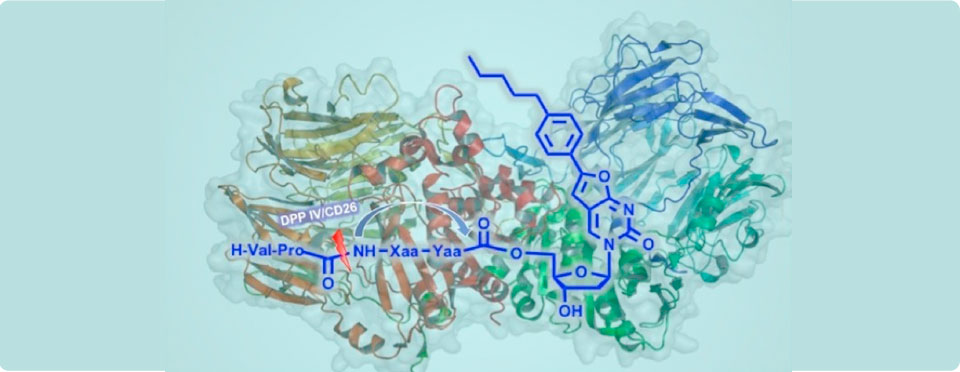
2013 - Diez-Torrubia, A.; Cabrera, S.; de Castro, S.; García-Aparicio, C.; Mulder, G.; De Meester, I.; Camarasa, M.J.; Balzarini, J.; Velázquez; S.
Novel water-soluble prodrugs of acyclovir cleavable by the dipeptidylpeptidase IV (DPP IV/CD26) enzyme. Eur. J. Med. Chem., 2013, 70, 456-468.
-
2013 - Toro, M. A.; Sánchez-Murcia, P. A.; Moreno, D.; Ruiz-Santaquiteria, M.; Alzate, J. F.;
Negri, A.; Camarasa, M. J.; Gago, F.; Velázquez, S.; Jiménez-Ruiz, A.
Probing the dimerization interface of Leishmania infantum trypanothione reductase with site-directed mutagenesis and short peptides. ChemBioChem, 2013, 14, 1212-1217.
-
2013 - Casanova, E.; Moreno, D.; Gigante, A.; Rico, E.; Genes, C. M.; Oliva, C.; Camarasa, M. J.; Gago, F.; Jiménez-Ruiz, A.; Pérez-Pérez, M. J.
5´-Trityl substituted thymidine derivatives as a novel class of antileishmanial agents: Leishmania infantum EndoG as a potential target. ChemMedChem, 2013, 8, 1161-1174
-
2013 - Quesada, E.; Flores, A.
Entry Inhibitors Directed Towards Glycoprotein gp120: An Overview on a Promising Target for HIV-1 Therapy. Curr. Med. Chem., 2013, 20, 751-771.
-
2013 - Carrero, P.; Ardá, A.; Alvarez, M.; Doyagüez, E. G.; Rivero-Buceta, E.; Quesada, E.; Prieto, A.; Solís, D.; Camarasa, M. J.; Peréz-Pérez, M. J.; Jiménez-Barbero, J.; San-Félix, A.
Differential recognition of mannose-based polysaccharides by tripodal receptors based on a triethylbenzene scaffold substituted with trihydroxybenzoyl moieties. Eur. J. Org. Chem., 2013,65–76
-
2013 - Camarasa, M. J.; Velázquez, S.; Sán-Félix, A.; Pérez-Pérez, M. J.
Synthesis of 3'-Spiro-substituted Nucleosides: Chemistry of TSAO Nucleoside Derivatives. en Chemical Synthesis of nucleoside analogues, P. Merino (ed) John Wiley & Sons, chapter 10, pages 427-472 (2013). ISBN 978-1-118-00751-8
-
2012 - Diez-Torrubia, A.; Cabrera, S.; De Meester, I.; Camarasa, M. J.; Balzarini, J.; Velázquez, S.
Dipeptidyl-peptidase IV-activated prodrugs of anti-Varicella Zoster Virus bicyclic nucleoside analogues containing different self-cleavage spacers systems. ChemMedChem, 2012, 7, 1612-1622.
-
2012 - Cortés-Cabrera, A.; Klett, J.; G. Dos Santos, H.; Perona, A.; Gil-Redondo, R.; Francis, S.; Priego, E. M.; Gago, F.; Morreale, A.
CRDOCK: an ultrafast muiltipurpose protein-ligand docking tool. J. Chem. Inf. Model., 2012, 52, 2300-2309.
-
2012 - Diez-Torrubia, A.; Cabrera, S.; Lambeir, A. M.; Balzarini, J.; Camarasa, M. J.; Velázquez, S.
Dipeptidyl peptidase IV (DPPIV/CD26)-based progrugs of hydroxy-containing drugs. ChemMedChem, 2012, 7, 618-628.
-
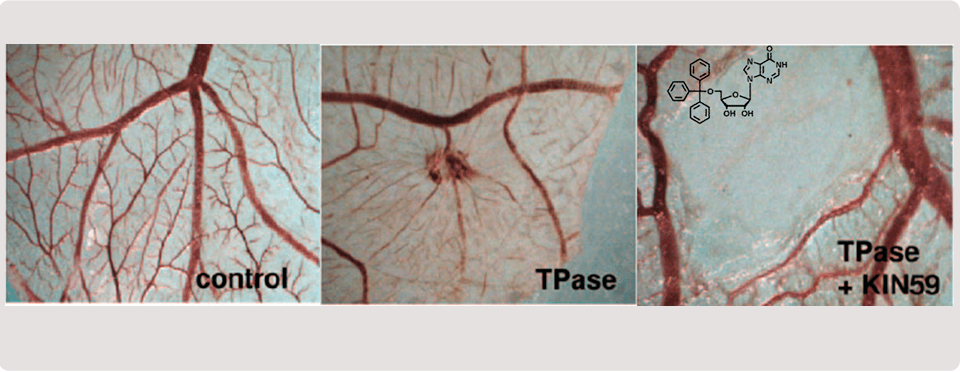
2012 - Liekens, S.; Bronckaers, A.; Belleri, M.; Bugatti, A.; Sienaert, R.; Ribatti, D.; Nico. B.; Gigante, A.; Casanova, E.; Opdenakker, G.; Pérez-Pérez, M. J.; Balzarini, J.; Presta, M.
The thymidine phosphorylase inhibitor 5´-O-tritylinosine (KIN59) is an antiangiogenic multitarget fibroblast growth factor-2 antagonist. Mol. Cancer Ther., 2012, 11, 817-829.
-
2012 - Priego, E. M.; Karlsson, A.; Gago, F.; Camarasa, M. J.; Balzarini, J.; Pérez-Pérez, M. J.
Recent advances in thymidine kinase 2 (TK2) inhibitors and new perspectives for potential applications. Curr. Pharm. Des., 2012, 18, 2981-2994.
-
2012 - Aguado, L.; Canela, M. D.; Thibaut, H. J.; Priego, E. M.; Camarasa, M. J.; Leyssen, P.; Neyts, J.; Pérez-Pérez, M. J.
Efficient synthesis and anti-enteroviral activity of 9-arylpurines. Eur. J. Med. Chem., 2012, 49, 279-288
-
2011 - Bonache, M. C.; Cordeiro, A.; Quesada, E.; Vanstreels, E.; Daelemans, D.; Camarasa, M. J.; Balzarini, J.; San Félix, A.
Selective inhibition of Human Immunodeficiency Virus type 1 (HIV-1) by a novel family of tricyclic nucleosides. Antivir. Res., 2011, 92, 37-44
-
2011 - Lozano, V.; Hernández, R.; Ardá, A.; Jiménez-Barbero, J.; Mijangos, C.; Pérez-Pérez, M. J.
An asparagines/tryptophan organogel showing a selective response towards fluoride anions. J. Mat. Chem., 2011, 21, 8862-8870
-
2011 - de Castro, S.; Familiar, O.; Andrei, G.; Snoeck, R.; Balzarini, J.; Camarasa, M. J.; S. Velázquez.
From β-amino-γ-sultone to unusual bicyclic pyridine and pyrazine heterocyclic systems: synthesis, cytostatic and antiviral activity ChemMedChem., 2011, 6, 686-697.
-
2011 - Diez-Torrubia, A.; Balzarini, J.; Andrei, G.; Snoeck, R.; De Meester, I.; Camarasa, M. J.; Velázquez, S.
Dipeptidyl peptidase IV dependent water-soluble prodrugs of highly liphopilic bicyclic nucleoside analogues J. Med. Chem., 2011, 54, 1927-1942.
-
2011 - Das, K.; Bauman, J. D.; Rim, A. S.; Dharia, Ch.; Clark Jr, A. D.; Camarasa, M. J.; Balzarini, J.; Arnold, E.
Crystal structure of TSAO in complex with HIV-1 reverse transcriptase redefines the elastic limits of the non-nucleoside inhibitor-binding pocket. J. Med. Chem., 2011, 54, 2727-2737.
-
2011 - Lozano, V.; Aguado, L.; Hoorelbeke, B.; Renders, M.; Camarasa, M. J.; Schols, D.; San-Félix, A.; Balzarini, J.; Pérez-Pérez, M. J.
Targeting HIV entry through interaction with envelope glycoprotein 120 (gp120): Synthesis and antiviral evaluation of 1,3,5-triazines with aromatic amino acids. J. Med. Chem., 2011, 54, 5335-5348.
-
2010 - Casanova, E.; Priego, E. M.; Jimeno, M. L.; Aguado, L.; Negri, A.; Gago, F.; Camarasa, M. J.; Pérez-Pérez, M. J.
Intramolecular cation-π interactions as the driving force to restrict the conformation of certain nucleosides. J. Org. Chem. 2010, 75, 1974-1981.
-
2010 - Diez-Torrubia, A.; García-Aparicio, C.; Cabrera, S.; De Meester, I.; Balzarini, J.; Camarasa, M. J.; Velázquez, S.
Application of the dipeptidyl-peptidase IV (DPPIV/CD26) based prodrug approach to different amine-containing drugs. J. Med. Chem. 2010, 53, 559-572.
-
2010 - Aguado, L.; Thibaut, H. J.; Priego, E. M.; Jimeno, M. L.; Camarasa, M. J.; Netyts, J.; Pérez-Pérez, M. J.
9-Arylpurines as a novel class of enterovirus inhibitors. J. Med. Chem. 2010, 53, 316-324.
-
2010 - Familiar,O.; Munier-Lehman, H.; Aínsa, J. A.; Camarasa, M. J.; Pérez-Pérez, M. J.
Design, synthesis and inhibitory activity against M. tuberculosis thymidine monophosphate kinase of acyclic nucleoside analogues with a distal imidazoquinolinone. Eur. J. Med. Chem. 2010, 45, 5910-5918.
-
2009 - Francois, K. O.; Pannecouque, C.; Auwerx, J.; Lozano. V.; Pérez-Pérez, M. J.; Schols, D.; Balzarini, J.
The phthalocyanine prototype derivative alcian blue is the first synthetic agent with selective anti-human immunodeficiency virus activity due to its gp120 glycan-binding potential. Antimicrob. Agents Chemother. 2009, 53, 4852-4859.
-
2009 - Bonache, M. C.; Cordeiro, A.; Quesada, E.; Camarasa, M. J.; Jimeno, M. L.; San-Félix, A.
One-pot synthesis of polycyclic nucleosides with unusual molecular skeletons J. Org. Chem. 2009, 74, 9071-9081.
-
2009 - de Castro, S.; García-Aparicio, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Camarasa, M. J.; Velázquez, S.
4-Benzyloxy-γ-Sultone Derivatives: Discovery of a Novel Family of Non-Nucleoside Inhibitors of Human Cytomegalovirus (HCMV) and Varicella-Zoster Virus (VZV). J. Med. Chem. 2009, 52, 1582-1591.
-
2009 - Lozano, V.; Hernández, R.; Mijangos, C.; Pérez-Pérez, M. J.
A novel organogelator incorporating tert-butyl esters of asparagines Org. Biomol. Chem. 2009, 7, 364-369.
-
2009 - Aguado, L.; Camarasa M. J.; Pérez-Pérez. M. J.
Microwave-assisted synthesis of 9-arylpurines J. Comb. Chem. 2009, 11, 210-212.
-
2009 - Bronckaers, A.; Aguado, L.; Negri, A.; Camarasa, M. J.; Balzarini, J.; Pérez-Pérez, M. J., Gago, F.; Liekens, S.
Identification of Asp203 in human thymidine phosphorylase as an important residue for both catalysis and non-competitive inhibition by the small “crystallization chaperone” 5´-O-tritylinosine (KIN59). Biochem. Pharmacol. 2009, 78 (3), 231-240.
-
2008 - de Castro, S.; Peromingo, M. T.; Naesens, L.; Andrei, G.; tSnoeck, R.; Balzarini, B.; Velázquez, S.; Camarasa, M. J.
4”-Benzoylureido-TSAO derivatives as Potent and Selective Non-Nucleoside HCMV Inhibitors. Structure-Activity Relationship and Mechanism of Antiviral Action. J. Med. Chem. 2008, 51, 5823-5832.
-
2008 - de Castro, S.; Peromingo, M. T.; Lozano, A. E; Camarasa, M. J.; Velázquez, S.
Reactivity of the 4-amino-1,2-oxathiole-2,2-dioxide heterocyclic system: A combined experimental and theoretical study. Chemistry-A European Journal, 2008, 14, 9620-9632.
-
2008 - Familiar, O.; Munier-Lehmann, H.; Negri, A.; Gago, F.; Douguet, D.; Rigouts, L.; Hernández, A. I.; Camarasa, M. J.; Pérez-Pérez, M. J.
Exploring acyclic nucleoside analogues as inhibitors of Mycobacterium tuberculosis thymidylate kinase ChemMedChem. 2008, 3, 1083-1093.
-
2008 - Pérez-Pérez, M. J.; Priego, E. M.; Hernández, A. I.; Familiar, O.; Negri, A., Gago, F.; Camarasa, M. J.; Balzarini, J.
Structure, physiological role and specific inhibitors of human thymidine kinase 2 (TK2): Present and future. Med. Res. Rev. 2008, 28, 797-820.
-
2008 - Priego, E. M.; Pérez-Pérez, M. J.; Von Frijtag Drabbe Kuenzel, J.; de Vries, H.; Ijzerman, A. P.; Camarasa, M. J.; Martín-Santamaría, S.
Selective human adenosine A3 antagonists based on pyrido[2,1-f]purine-2,4-diones: novel features of hA3 antagonist binding. ChemMedChem. 2008, 3, 111-119.
-
2007 - Cordeiro, A.; Jimeno, M. L.; Maestro, M. A.; Camarasa, M. J.; Quesada, E.; San-Felix, A.
Synthesis of highly condensed polycyclic carbohydrates by reaction of a spirocyclic enamino sulfonate derived from D-xylofuranose with bifunctional reagents. J. Org. Chem. 2007, 72, 9713-9721.
-
2007 - Casanova, E.; Pérez-Pérez, M. J.; Kappe, C.O.
Microwave-Assisted Selective 5’-O-Trityl Protection of Inosine Derivatives. Synlett. 2007, 1733-1735.
-
2007 - Liekens, S.; Bronckaers, S.; Pérez-Pérez, M. J.; Balzarini, J.
Targeting platelet-derived endothelial cell growth factor/thymidine phosphorylase for cancer therapy. Biochem. Pharmacol. 2007, 74, 1555-1567.
-
2007 - McGuigan, C.; Pathirana, R. N.; Migliore, M.; Adak, R.; Luoni, G.; Jones, A. T.; Diez-Torrubia, A.; Camarasa, M. J.; Velázquez, S.; Henson, G.; Snoeck, R.; Andrei, G.; Balzarini.
Preclinical Development of bicyclic nucleoside analogues as potent and selective inhibitors of varicella zoster virus. J. Antimicrob. Chemother. 2007, 60, 1316-1330.
-
2007 - García-Aparicio, C.; Diez-Torrubia, A.; Balzarini, J.; Lambeir, A-M.; Velázquez, S.; Camarasa, M. J.
Efficient conversion of tetrapeptide-based TSAO prodrugs to the parent drug by dipeptidyl-peptidase IV (DPPPIV/CD26). Antiviral Res. 2007, 76, 130-139.
-
2006 - Cordeiro, A; Quesada, E; Bonache, M. C; Velázquez, S; Camarasa, M. J; San-Félix, A.
A cyclic enamine derived from 1,2-O-isopropylidene-α-D-xylofuranose as a novel carbohydrate intermediate to achieve skeletal diversity. J. Org. Chem. 2006, 71, 7224-7235.
-
2006 - Sluis-Cremer, N; Hamamouch, N; San-Félix, A; Velázquez, S; Balzarini, J; Camarasa, M. J.
Structure-activity relationships of [2',5'-bis-(O-tert-butyldimethylsilyl)-ß-D-ribofuranosyl]-3'-spiro-5''-(4''-amino-1'',2''-oxathiole- 2'',2''-dioxide) thymine (TSAO-T) derivatives as inhibitors of HIV-1 reverse transcriptase dimerization. J. Med Chem. 2006, 49, 4834-4841.
-
2006 - de Castro, S; García-Aparicio, C; Van Laethen, K; Lobatón, E; De Clercq, E; Balzarini, J; Camarasa, M. J; Velázquez, S;
Discovery of TSAO derivatives with an unusual HIV-1 activity/resistance profile. Antivir. Res. 2006, 71, 15-23.
-
2006 - Camarasa, M. J; Velázquez, S; San-Félix, A; Pérez-Pérez, M. J; Gago, F.
Dimerization of HIV-1 reverse transcriptase, protease and integrase: a single mode of inhibition for the three enzymes?. Antivir. Res. 2006, 71, 260-267.
-
2006 - de Castro, S; Lozano, A; Jimeno, M. L; Pérez-Pérez, M. J; San Félix, A; Camarasa, M. J; Velázquez, S.
Unprecedented lability of 5'-O-tert-butyldimethylsilyl group from 3'-spiro-5''-(4''-acylamino-1'',2''-oxathiole- 2'',2''-dioxide) nucleoside derivatives via neighboring group participation of the 4''-acylamino residue. J. Org. Chem. 2006, 71, 1407-1415.
-
2006 - Camarasa, M. J.; Velázquez, S.; San-Félix, A.; Pérez Pérez, M. J.; Bonache, M. C., de Castro, S.
TSAO derivatives, inhibitors of HIV-1 reverse transcriptase dimerization: recent progress. Curr. Pharm. Des. 2006, 12, 1895-1907.
-
2006 - Casanova, E; Hernández, A. I; Priego, E. M; Liekens, S; Camarasa, M. J; Balzarini, J; Pérez-Pérez, M. J.
5'-O-Tritylinosine and Analogues as Allosteric Inhibitors of Human Thymidine Phosphorylase. J. Med. Chem. 2006, 49, 5562-5570.
-
2006 - Liekens, S.; Bronckaers, A.; Hernández, A. I.; Priego, E. M.; Casanova, E.; Camarasa, M. J.; Pérez-Pérez, M. J.; Balzarini, J.
5’-O-tritylated nucleoside derivatives: inhibition of thymidine phosphorylase and angiogenesis. Mol Pharmacol. 2006, 70, 501-509.
-
2006 - El Omari, K; Bronckaers, A; Liekens, S; Pérez-Pérez, M. J; Balzarini, J; Stammers, D. K.
Structural basis for non-competitive product inhibition in human thymidine phosphorylase: implications for drug design. Biochem. J. 2006, 399, 199-204.
-
2006 - García Aparicio, C; Bonache, M. C; De Meester, I; San-Félix, A; Balzarini, J; Camarasa, M. J; Velázquez, S.
Design and Discovery of a novel dipeptidil-peptidase IV (CD26)-based prodrug approach. J. Med. Chem. 2006, 49, 5339-5351.
-
2006 - Hernández, A. I; Familiar, O; Negri, A; Rodríguez-Barrios, F; Gago, F; Karisson, A; Camarasa, M. J; Balzarini, J; Pérez-Pérez, M. J.
N1 -Substituted Thymine derivatives as mitochondrial thymidine Kinase (TK-2) inhibitors. J. Med. Chem. 2006, 49, 7766-7773.
-
2006 - Franzolin, E.; Rampazzo, C.; Pérez-Pérez, M. J.; Hernández, A. I.; Balzarini, J.; Bianchi, V.
Bromovinyl-deoxyuridine: a selective substrate for mitochondrial thymidine kinase in cell extracts. Biochem. Biophys. Res. Commun. 2006, 344, 30-36.
-
2005 - Bonache, M. C.; Chamorro, C.; Velázquez, S.; De Clercq, E.; Balzarini, J.; Rodríguez Barrios, F.; Gago, F.; Camarasa, M. J; San-Félix, A.
Improving the antiviral efficacy and selectivity of HIV-1 Reverse Transcriptase inhibitor TSAO-T by the introduction of functional groups at the N-3 position. J. Med. Chem. 2005, 48, 6653-6660.
-
2005 - Balzarini, J.; Auwerx, J.; Rodríguez-Barrios, F.; Chedad, A.; Farkas, V.; Ceccherini-Silberstein, F.; García-Aparicio, C.; Velázquez, S.; De Clercq, E.; Perno, C. F.; Camarasa, M. J.; Gago, F.
The amino acid Asn136 in HIV-1 reverse transcriptase (RT) maintains efficient association of both subunits and enables the rational design of novel RT inhibitors. Mol. Pharmacol. 2005, 68, 49-60.
-
2005 - de Castro, S., Lobatón, E., Pérez-Pérez, M. J., San-Félix, A., Cordeiro, A., Andrei, G., Snoeck, R., De Clercq, E., Balzarini, J., Camarasa, M. J., Velázquez, S.
Novel [2',5'-Bis-O-(tert-butyldimethylsilyl)-ß-D-ribofuranosyl]-3'-spiro-5"-(4"-amino-1",2"-oxathiole-2",2"-dioxide) derivatives with anti-HIV-1 and anti-Human-Cytomegalovirus activity. J. Med. Chem. 2005, 48, 1158-1168.
-
2005 - Auwerx, J.; Van Nieuwenhove; Rodríguez-Barrios, F.; De Castro, S.; Velázquez, S.; Ceccherini-Silberstein, F.; De Clercq, E.; Camarasa, M. J.; Perno, C. F.; Gago, F.; Balzarini, J.
The N137 and P140 amino acids in the p51 and the P95 amino acid in the p66 subunit of human immunodeficiency virus type 1 reverse transcriptase (RT) are instrumental to maintain the polymerase activity and are prime targets for the rational design of new classes of anti HIV-1 RT drugs. FEBS Lett. 2005, 579, 2294-2300.
-
2005 - Camarasa, M. J.; Velázquez, S.; San-Félix, A.; Pérez Pérez, M.J.
TSAO derivatives, the first non-peptide inhibitors of HIV-1 RT dimerization. Antiviral. Chem. Chemother. 2005, 16, 147-153.
-
2005 - Auwerx, J.; Rodríguez-Barrios, F.; Ceccherini-Silberstein, F.; San Félix, A.; Velázquez, S.; De Clercq, E.; Camarasa, M. J.; Perno, C. F.; Gago, F.; Balzarini, J.
Role of Thr139 in the human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) sensitivity to (+)-calanolide A. Mol. Pharmacol. 2005, 68, 652-659.
-
2005 - Pérez-Pérez, M. J; Hernández, A. I.; Priego, E. M.; Rodríguez-Barrios, F.; Gago, F; Camarasa, M. J; Balzarini, J.
Mitochondrial Thymidine Kinase Inhibitors. Curr. Top. Med. Chem., 2005, 5, 1205-1220.
-
2005 - Pérez-Pérez, M. J.; Priego, E, M.; Hernández, A. I.; Camarasa, M. J.; Balzarini, J.; Liekens, S.
Thymidine Phosphorylase Inhibitors: Recent developments and potential therapeutic applications. Mini Rev. Med. Chem. 2005, 5, 1113-1124.
-
2004 - Bonache, M. C.; Chamorro, C.; Cordeiro, A.; Camarasa, M. J.; Jimeno M. L. y San-Félix, A.
Synthesis of novel Bi-, Tri-, and Tetracyclic nucleosides by reaction of a common cyclic enamine derived from TSAO-T with nucleophiles J. Org. Chem. 2004, 69, 8758-8766.
-
2004 - Camarasa, M. J.; San-Félix, A.; Velázquez, S.; Pérez-Pérez, M. J.; Gago, F.; Balzarini, J.
TSAO compounds: The comprehensive story of a unique family of HIV-1 specific inhibitors of Reverse Transcriptase. Curr. Top. Med. Chem. 2004, 4, 945-963.
-
2004 - Velázquez, S.; Lobatón, E.; De Clercq, E.; Koontz, D. L.; Mellors, J. W.; Balzarini, J. y Camarasa, M. J.
Hybrids [TSAO-T]-[Foscarnet]: The first conjugate of foscarnet with a non-nucleoside reverse transcriptase inhibitor through a labile covalent ester bond J. Med. Chem. 2004, 47, 3418-3426.
-
2004 - Mendieta, J.; Martín-Santamaría, S.; Priego, E. M.; Balzarini, J.; Camarasa, M. J.; Pérez-Pérez, M. J.; Gago, F.
Role of His-85 in the Catalytic Mechanism of Thymidine Phosphorylase as Assessed by Targeted Molecular Dynamics Simulations and Quantum Mechanical Calculations. Biochemistry, 2004, 43, 405-414.
-
2004 - Liekens, S.; Hernández, A. I.; Ribatti, D.; De Clercq, E.; Camarasa, M. J.; Pérez-Pérez, M. J.; Balzarini, J.
The nucleoside derivative 5´-O-trityl-inosine (KIN59) suppresses thymidine-phosphorylase triggered angiogenesis via a non-competitive mechanism of action. J. Biol. Chem. 2004, 279, 29598-29605.
-
2004 - Priego, E. M.; Balzarini, J.; Karlsson, A.; Camarasa, M. J.; Pérez-Pérez; M. J.
Synthesis and evaluation of thymine-derived carboxamides against Mitochondrial Thymidine Kinase (TK-2) and related enzymes. Bioorg. Med. Chem. 2004, 12, 5079-5090.
-
2003 - Chamorro, C.; Luengo, S. M.; Bonache, M. C.; Velázquez, S.; Pérez-Pérez, M. J.; Camarasa, M. J.; Gago, F.; Jimeno M. L.; San-Félix, A.
Synthesis and Structural Characterization of Pyrimidine Bi- and Tricyclic Nucleosides with Sugar Puckers Conformationally Locked into the Eastern Region of the Pseudorotational Cycle. J. Org. Chem. 2003, 68, 6695-6704.
-
2003 - Bonache, M. C.; Chamorro, C.; Lobatón, E.; De Clercq, E.; Balzarini, J.; Velázquez, S.; Camarasa, M. J.; San-Félix, A.
Structure-activity relationship studies on a novel family of specific HIV-1 reverse transcriptase inhibitors. Antiviral. Chem. Chemother. 2003, 14, 249-262.
-
2003 - Hernández, A. I.; Balzarini, J.; Rodríguez-Barrios, F.; San-Félix, A.; Karlsson, A.; F. Gago, F.; Camarasa, M. J.; Pérez-Pérez, M. J.
Improving the Selectivity of Acyclic Nucleoside Analogues as Inhibitors of Human Mitochondrial Thymidine Kinase: Replacement of a Triphenylmethoxy Moiety with Substituted Amines and Carboxamides. Bioorg. Med. Chem. Lett., 2003, 13, 3027-3030.
-
2003 - Balzarini, J.; Hernández, A. I.; Roche, P.; Esnouf, R.; Karlsson, A.; Camarasa, M. J.; Pérez-Pérez, M. J.
Non-nucleoside inhibitors of mitochondrial thymidine kinase (TK-2) differentially inhibit the closely related herpes simplex virus type 1 TK and Drosophila melanogaster multifunctional deoxynucleoside kinase. Mol. Pharmacol., 2003, 63, 263-270.
-
Lobatón, E.; Rodríguez-Barrios, F.; Gago, F.; Pérez-Pérez, M. J.; De Clercq, E.; Balzarini, J.; Camarasa, M. J.; Velázquez, S.
Synthesis of 3"-substituted TSAO derivatives with anti-HIV-1 and HIV-2 activity through an efficient palladium-catalyzed cross-coupling approach. J. Med. Chem. 2002, 45, 3934-3945.
-
Priego, E. M.; Von Frijtag Drabbe Kuenzel, J.; Ijzerman, A. P.; Camarasa, M. J.; Pérez-Pérez, M. J.
Pyrido[2,1-f]purine-2,4-dione derivatives as novel class of highly potent human A3 adenosine receptor antagonists. J. Med. Chem. 2002, 45, 3337-3344.
-
Hernández, A. I.; Balzarini, J.; Karlsson, A.; Camarasa, M. J.; Pérez-Pérez, M. J.
Acyclic nucleoside analogues as novel inhibitors of human mitochondrial thymidine kinase. J. Med. Chem. 2002, 45, 4254-4263.
-
Rodríguez-Barrios, F.; Pérez, C.; Lobatón, E.; Velázquez, S.; Chamorro, C.; San-Félix, A.; Pérez-Pérez, M. J.; Camarasa, M. J.; Pelemans, H.; Balzarini, J.; Gago, F.
Identification of a putative binding site for TSAO derivatives at the p51/p66 interface of HIV-1 reverse transcriptase. J. Med. Chem. 2001, 44,1853-1865.
-
Menéndez-Arias, L.; Abraha, A.; Quiñones-Mateu, M. E.; Mas, A.; Camarasa , M. J.; Arts, E. J.
Functional characterization of chimeric reverse transcriptases with polypeptide subunits of highly divergent group M and O HIV-1 strains. J. Biol. Chem. 2001, 276, 27470-27479.
-
Sluis-Cremer, N.; Dmitrienko, G. I.; Balzarini, J. ; Camarasa, M. J.; Parniak, M. A.
Human immunodeficiency virus type 1 reverse transcriptase dimer destabilization by 1-spiro[4"-amino-2",2"-dioxo-1",2"-oxathiole-5",3'-[2',5'-bis-O-(tert-butyldimethylsilyl)-β-ribofuranosyl]]-3-ethylthymine. Biochemistry 2000, 39, 1427-1433.
-
Esteban-Gamboa, A.; Balzarini, J.; Esnouf, R.; De Clercq, E.; M. J. Camarasa, M. J.; Pérez-Pérez, M. J.
Design, synthesis and enzymatic evaluation of multisubstrate analogue inhibitors of Escherichia coli thymidine phosphorylase. J. Med. Chem. 2000, 43, 971-983.
-
Van Laethem, K.; Scmit, J. C.; Balzarini, J.; Witvrouw, M.; Pérez, M. J.; Camarasa, M. J.; Esnouf, R. M.; Aquaro, S.; Cenci, A.; Perno, C. F.; Hermans, P.; Sprecher, S.; Ruiz, L.; Clotet, B.; Wijngaerden, E.; Ranst, M. V.; Desmiter, J.; De Clercq, E.; Vandamme, A. M.
Presence of TSAO-resistant virus strains in TSAO-unexperienced patients. AIDS Res. Human Retrovir. 2000, 16, 825-833.
-
Velázquez, S.; Tuñón, V.; Jimeno, M. L.; Chamorro, C.; De Clercq, E.; Balzarini, J.; Camarasa, M. J.
Potential Multi-functional inhibitors of HIV-1 reverse transcriptase. Novel [AZT]-[TSAO-T] and [d4T]-[TSAO-T] heterodimers modified in the linker and in the dideoxynucleoside region. J. Med. Chem. 1999, 42, 5188-5196.
-
Velázquez, S.; Chamorro, C.; Pérez-Pérez, M. J.; Alvarez, R.; Jimeno, M. L.; Martín Domenech, A.; Pérez, C.; Gago, F.; De Clercq, E.; Balzarini, J.; San-Félix, A.; Camarasa, M. J.
Abasic analogues of TSAO-T as the first sugar derivatives that specifically inhibit HIV-1 reverse transcriptase. J. Med. Chem. 1998, 41, 4636-4647.
-
Balzarini, J.; Esteban-Gamboa, A.; Esnouf, R.; Liekens, S.; Neyts, J.; De Clercq, E.; Camarasa, M. J.; Pérez-Pérez, M. J.
7-Deazaxanthine, a novel prototype inhibitor of thymidine phosphorylase. FEBS Letters 1998, 438, 91-95.
-
Velázquez, S., Alvarez, R., Pérez, C., Gago, F., de Clercq, E. Balzarini, J., Camarasa, M. J.
Regiospecific synthesis and anti-human immunodeficiency virus type 1 activity of novel 5-substituted N-alkylcarbamoyl and N,N-dialkyl carbamoyl 1,2,3-triazole-TSAO analogues as Inhibitors of HIV-1 Reverse Transcriptase. Antiviral. Chem. Chemother. 1998, 9, 481-489. Portada del número
-
Velázquez, S.; Alvarez, R.; San-Félix, A.; Jimeno, M. L.; De Clercq, E.; Balzarini, J.; Camarasa, M. J.
Synthesis and anti-HIV activity of [AZT]-[TSAO-T] and [AZT]-[HEPT] dimers as potential multifunctional inhibitors of HIV-1 reverse transcriptase. J. Med. Chem. 1995, 38, 1641-1649.
-
Balzarini, J.; Pérez Pérez, M. J.; Velázquez, S.; San Félix, A.; Camarasa, M. J.; De Clercq, E.; Karlsson, A.
Suppression of the breakthough of human immunodeficiency virus type 1 (HIV-1) in cell culture by thiocarboxanilide derivatives when used individually or in combination with other HIV-1-specific inhibitors (i.e. TSAO derivatives). Proc. Natl. Acad. Sci. USA, 1995, 92, 5470-5475.
-
Alvarez, R.; Velázquez, S.; San Félix, A.; Aquaro, S.; De Clercq, E.; Perno, C. F.; Karlsson, A.; Balzarini, J.; Camarasa, M. J.
1,2,3-Triazole-[2',5'-Bis-O-(tert-butyldimethylsilyl)-β-D-ribofuranosyl]-3'-spiro-5"-(4"-amino-1",2"-oxathiole-2",2"-dioxide) (TSAO) Analogues: Synthesis and anti-HIV-1 activity. J. Med. Chem. 1994, 37, 4185-4194.
-
Jonckheere, H.; Taymans, J. M.; Balzarini, J.; Velázquez, S.; Camarasa, M. J.; Desmyter, J.; De Clercq, E.; Anné, J.
Resistance of HIV-1 Reverse Transcriptase against [2',5'-bis-O-tert-butyldimethylsilyl)-3'-spiro-5"-(4"-amino-1",2"-oxathiole-2",2"-dioxide)] (TSAO) derivatives is determined by the mutation Glu138ÆLys on the p51 subunit. J. Biol. Chem. 1994, 269, 25255-25258.
-
Velázquez, S.; Jimeno, M. L.; Huss, S.; Camarasa, M. J.
Synthesis and NMR Conformational Studies of γ-butyrolactones of Nucleosides as Chiral Synthons for the Preparation of 2'-C- and 3'-C-branched-chain Nucleosides. J. Org. Chem. 1994, 59, 7661-7670.
-
Balzarini, J.; Pérez Pérez, M. J.; San Félix, A.; Schools, D.; Perno, C.-F.; Vandame, A.; Camarasa, M. J.; De Clercq, E.
2',5'-Bis-O-(tert-butyldimethylsilyl)-3'-spiro-5"-(4"-amino-1",2"-oxathiole-2",2"-dioxide) pyrimidine nucleoside analogues: Novel higly selective inhibitors of HIV type 1 that are targeted at the viral RT. Proc. Natl. Acad. Sci. USA, 1992, 89, 4392-4396. (Comentado en ChemTracks-Org. 1993, 6, 67).
-
Pérez Pérez, M. J.; San Félix, A.; Balzarini, J.; De Clercq, E.; Camarasa, M. J.
TSAO Analogues. Stereospecific synthesis and anti-HIV-1 activity of 1-[2',5'-bis-O-(tert-butyl-dimethylsilyl)-β-D-ribofuranosyl]-3'-spiro-5"-(amino-1",2"-oxathiole-2",2"-dioxide) pyrimidine and pyrimidine modified nucleosides. J. Med. Chem. 1992, 35, 2988-2995.