Our research focuses on the design and development of new drugs, and pharmacological tools, aimed at the treatment and diagnosis of neurodegenerative diseases and cancer. We use modern organic synthesis methodologies, as well as innovative tools and concepts from medical chemistry research, for its later application in medicine.
Our group works in the development of new selective radiotracers for the in vivo diagnosis of taoupathies by means of positron emission tomography 18F-PET. Tauopathies are neurodegenerative diseases characterized by the accumulation of tau protein in neurons. The most popular is Alzheimer’s disease (AD), although there are other tauopathies such as Pick’s disease and progressive supranuclear palsy. At present, the diagnosis of AD can only be confirmed by postmortem analysis after autopsy. There is, therefore, the need to investigate and develop new methodologies for early diagnosis of AD, which contribute to a greater efficacy in the development of new drugs modulating the disease.
AD is characterized at the pathological level mainly by the presence of two different aggregates of protein type in the brain: β-amyloid, the accumulation of which forms the so-called senile plaques (SP) in the extracellular medium and hyperphosphorylated tau, deposited intracellularly in the form of Neurofibrillary tangles (NFT). Due to the coexistence of both SP and NFT in the brains of patients with AD, both can be considered biomarkers of the disease. The function of the tau protein, once phosphorylated, is the assembly and stabilization of microtubules, filaments that have a structural function, and which in neurons are specifically related to dendritic ramifications. When tau is hyperphosphorylated, an aggregation process begins which results in insoluble helical filaments (NFT), losing its stabilizing function of the microtubules. This destabilization finally causes neuronal death and therefore, the interruption of the synapse with the consequent loss of memory in patients.
Currently, the most developed PET radiotracers are those corresponding to β-amyloid. Some of these radiotracers developed for SPs have also shown some ability to detect NFTs, but their differential specificity between SPs and NFTs is low. On the other hand, recent studies indicate that the accumulation of tau is related to the appearance of the first symptoms of AD and that the density of the neurofibrillary tangles is associated with the progression of the disease, in contrast to β-amyloid. In addition, the appearance of NFT in different and specific areas of the brain is an indicator of the degree of progression in AD. This fact allows to differentiate between the different tauopathies. Therefore, in the last years, tau has become an important therapeutic target for the detection of AD.
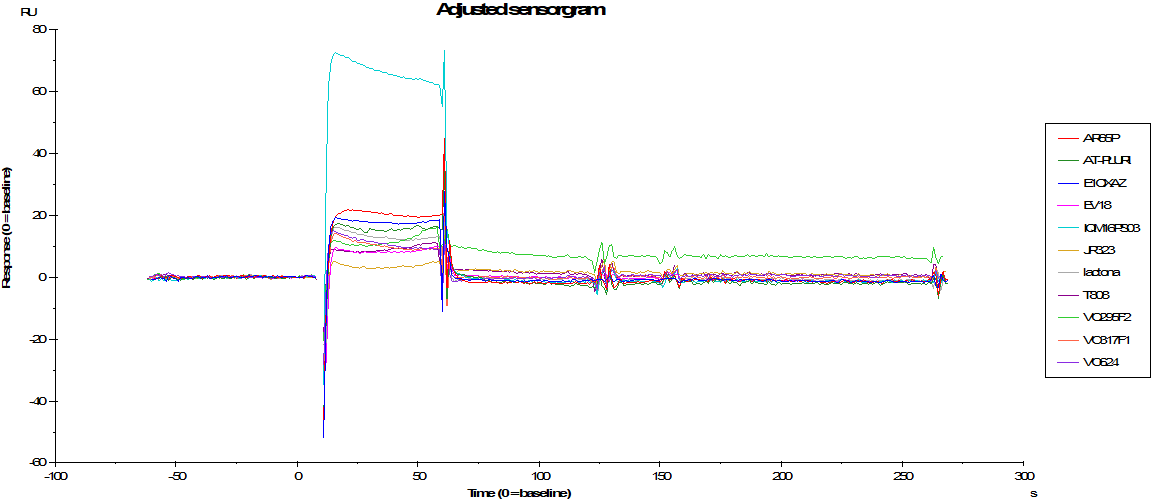
Our strategy is based, therefore, on the synthesis of new compounds of heterocyclic nature, with a selective affinity towards the aggregates of tau. With this purpose, we designe and synthesize the new chemical structures and evaluate them against tau aggregates using SPR technique, in vitro biological studies and NMR testing, selecting those compounds with the best affinities. Subsequently we perform the radiosynthesis of the corresponding 18F-labeled products and evaluate in vivo their potential as radiotracers by positron emission tomography (18F-PET). Additionally we perform the pharmacological evaluation of these compounds, including in vivo affinity and selectivity assays to NFTs, lipophilicity, toxicity and metabolism studies.
On the other hand, we are interested in the discovery of drugs that act selectively on cancer stem cells (CSC) in order to develop new therapeutic tools against breast cancer and gliomas, mainly.
The enormous intra and intertumoral variability is the main cause of therapeutic failure and highlights the absence of specific antitumor therapies for each type and subtype of cancer disease. The phenotypic and functional heterogeneity existing between the different cancers occurs not only at the tumors found in different tissues, but even among those in the same organ (intertumoral heterogeneity). The surprising scenario of tumor heterogeneity is greatly complicated by the fact that these variations also occur within the same tumor (intratumoral heterogeneity). Numerous research studies in recent years have demonstrated a clear relationship between the pathways that control the self-renewal capacity of tumor cells and the etiology of the oncologic disease itself.
Nowadays, the Cancer Stem Cells (CSC) model is the main model to explain how tumors originate, maintain and expand, as well as their mechanisms to evade the treatments giving rise to relapses or metastasis. This model postulates that only a cellular subpopulation, with the characteristics of self-renewal and differentiation attributed to stem cells, would be able to generate and maintain the tumor.
The presence of CSC in most major cancers appears to be one of the main causes of the failure of current cancer therapies both in terms of prevention of tumor progression and in the occurrence of relapses. Therefore, the elimination of this cell subpopulation by the use of drugs that act selectively and effectively on it is an essential step to obtain an effective cure.
Additionally, numerous research studies have demonstrated the existence of a clear relationship between the various transcription factors that activate tissue hypoxia and the pathways that control the self-renewal capacity of cells, thus suggesting an essential role of Hypoxia Inducible Factor (HIF) in oncogenesis through the generation or expansion of CSCs. Therefore, the biology of HIF proteins has evolved in the last decade from their role in tumor angiogenesis to the etiology of oncologic disease itself.
The strategy of our research group focuses on the synthesis of non-toxic compounds that inhibit the activity of HIF and SOX-2 interfering with the formation of oncospheres, tumor cells that under culture conditions lose adhesion forming three-dimensional spheres which are admitted as CSCs capable of initiating tumors in animal models of cancer disease. The transcription factor SOX-2, regulated by HIF2α, is an embryonic marker that is associated to the most undifferentiated forms of breast cancer and gliomas, being in turn the functional responsible of the formation of oncospheres in cultures of tumor cell lines in vitro.