Nitroheterocycles as antiparasitic drugs
The active collaboration of our team with Profs. A. Gómez Barrio and J. A. Escario (Department of Parasitology, Faculty of Pharmacy, UCM), Y. Marrero and A. Meneses (Central University of Las Villas, Cuba), C. Olea (University of Chile), M. Sánchez Moreno (University of Granada) and R. Mondragón (CINVESTAV, México), has led to the discovery of several nitroheterocyclic compounds showing remarkable in vivo and/or in vitro activities against different protozoan parasites. We can mention the activities of some indazole and quinoxaline derivatives against Trypanosoma cruzi, Trichomonas vaginalis, Leishmania spp., and Toxoplasma gondii.
Discovery of nitroheterocycles with antichagasic activity
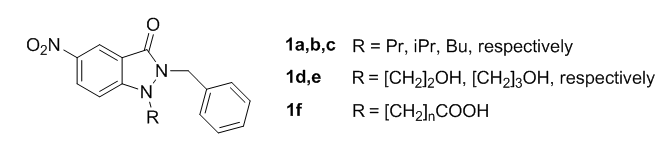
A phenotypic (whole cell) screening of our library of nitroheterocycles showed that 2-benzyl-1-methyl-5-nitroindazolin-3-one is a very active lead compound against T. cruzi. Structure-activity relationship studies explored the effect on activity of (a) introducing different alkyl, aryl and arylalkyl groups at position 2 of indazolinone ring, and (b) introducing benzyl moieties with substituents of different nature at position 2. In this step, the most active compounds were 1a–c (Figure 5) (IC50 = 1-2 μM for epimastigotes of T. cruzi, similar to that of standard antichagasic drug nifurtimox) (Vega et al., Eur. J. Med. Chem. 2012, 58, 214-227).
A major drawback of the mentioned indazole derivatives is their very low water solubility and their poor pharmacokinetic properties. We have observed that indazolinones do not support much variation at position 2 but they accept a large number of differently substituted groups at position 1 without considerable loss of activity. We are currently working on different synthetic strategies to improve the pharmacokinetic properties of these leads.
For instance, we have prepared derivatives containing ω-hydroxyalkyl chains at positions 1 of the indazole ring; these compounds (e.g. 1d,e) are very active but also rather insoluble in water. On the other hand, derivatives such as 1f, containing carboxylic acids moieties at position 1 are water-soluble salts but, unfortunately, they display poor antiparasitic activity (Fonseca-Berzal et al., Eur. J. Med. Chem. 2016, 115, 295-310). Currently, we are working in the preparation of derivatives containing ω-aminoalkyl chains at position 1 of indazolinone ring.
Discovery of nitroindazoles active against Leishmania spp., Trichomonas vaginalis, and Toxoplasma gondi
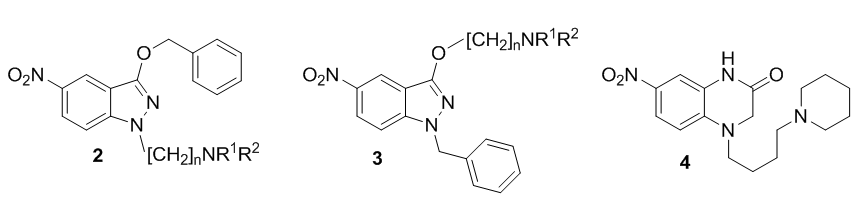
We have discovered that compounds 1 as well as 2 are also active against Leishmania spp. (Arán et al., unpublished results; Marín et al., Acta Trop. 2015, 148, 170-178), while indazoles 3 are, up to now, the best trichomonacidal compounds we have found (Arán et al., unpublished results). On the other hand, quinoxalinones such as 4 are effective against Toxoplasma gondii (Rivera Fernández et al., Parasitol. Res. 2016, 115, 2081-2096).
Mechanism of action studies
Nitro derivatives active against protozoan parasites act, via an initial metabolic reduction, through the final production of reactive oxygen species (ROS) or electrophilic metabolites able to damage essential biomolecules of parasites such as thiols, lipids, proteins, DNA, etc.
It has been proposed that the interference with glycosomal or mitochondrial enzymes involved in the catabolism of these parasites could contribute to the activity of some 5-nitroindazoles (Muro et al., Eur. J. Med. Chem. 2014, 74, 124-134). However, the precise mode of action of our indazole and quinoxaline derivatives needs to be investigated more thoroughly. In this context, we are working on the design of fluorescent probes to label the parasites’ organelles targeted by these compounds.
