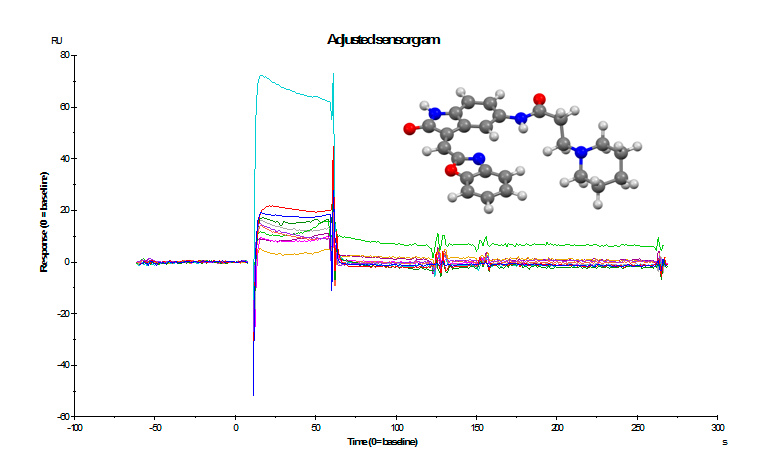
Synthesis and development of new PET radiotracers for the in vivo diagnosis of Alzheimer’s disease and other tauopathies.
Tauopathies are neurodegenerative diseases characterized by the accumulation of tau protein in neurons. The most popular is Alzheimer’s disease (AD), although there are other tauopathies such as Pick’s disease and progressive supranuclear palsy. At present, the diagnosis of AD can only be confirmed by postmortem analysis after autopsy. There is, therefore, the need to investigate and develop new methodologies for early diagnosis of AD, which contribute to a greater efficacy in the development of new drugs modulating the disease.
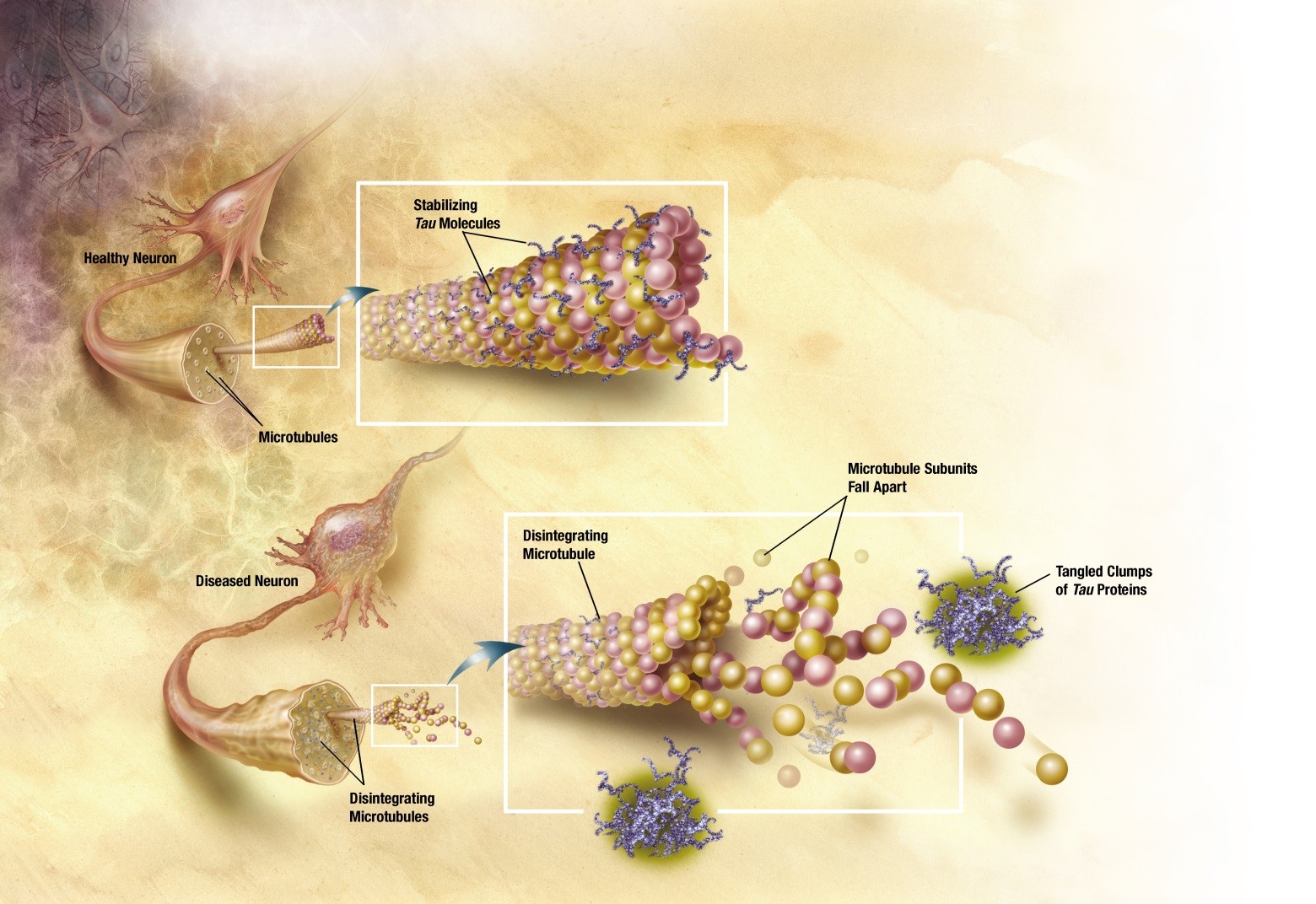
AD is characterized at the pathological level mainly by the presence of two different aggregates of protein type in the brain: β-amyloid, the accumulation of which forms the so-called senile plaques (SP) in the extracellular medium and hyperphosphorylated tau, deposited intracellularly in the form of Neurofibrillary tangles (NFT). Due to the coexistence of both SP and NFT in the brains of patients with AD, both can be considered biomarkers of the disease. The function of the tau protein, once phosphorylated, is the assembly and stabilization of microtubules, filaments that have a structural function, and which in neurons are specifically related to dendritic ramifications. When tau is hyperphosphorylated, an aggregation process begins which results in insoluble helical filaments (NFT), losing its stabilizing function of the microtubules. This destabilization finally causes neuronal death and therefore, the interruption of the synapse with the consequent loss of memory in patients.
Currently, the most developed PET radiotracers are those corresponding to β-amyloid. Some of these radiotracers developed for SPs have also shown some ability to detect NFTs, but their differential specificity between SPs and NFTs is low. On the other hand, recent studies indicate that the accumulation of tau is related to the appearance of the first symptoms of AD and that the density of the neurofibrillary tangles is associated with the progression of the disease, in contrast to β-amyloid. In addition, the appearance of NFT in different and specific areas of the brain is an indicator of the degree of progression in AD. This fact allows to differentiate between the different tauopathies. Therefore, in the last years, tau has become an important therapeutic target for the detection of AD.
Our strategy is based, therefore, on the synthesis of new compounds of heterocyclic nature, with a selective affinity towards the aggregates of tau. With this purpose, we designe and synthesize the new chemical structures and evaluate them against tau aggregates using SPR technique, in vitro biological studies and NMR testing, selecting those compounds with the best affinities. Subsequently we perform the radiosynthesis of the corresponding 18F-labeled products and evaluate in vivo their potential as radiotracers by positron emission tomography (18F-PET). Additionally we perform the pharmacological evaluation of these compounds, including in vivo affinity and selectivity assays to NFTs, lipophilicity, toxicity and metabolism studies.
This project is carried out in collaboration with Prof. Dr. Aurelio García Csaky and Prof. Dr. Miguel Angel del Pozo, Full Professors of the Complutense University of Madrid, both in the Pluridisciplinary Institute (CTQ2014-52213-R). (http://www.ucm.es/ip-1/osap-index)
References:
- Iqbal, K.; Liu, F.; Gong, C-X. Rev. Neurol. 2016, 12, 15-27.
- Okamuraa, N.; Haradab, R.; Furukawac, K.; Furumotod, S.; Tagoc, T.; Yanaia, K.; Araic, H.; Kudo Y. Ageing Research Reviews 2016, 30, 107-113.
- Sánchez-Sancho, F.; Csákÿ, A. G. Synthesis 2016, 48, 2165-2177.
- Roscales, S.; Sánchez, F.; Csákÿ, A. G. J. Org. Chem. 2015, 1754-1763.