Innovative drugs against Chagas disease
Chagas disease, also known as American trypanosomiasis, is caused by infection with the protozoan parasite Trypanosoma cruzi. The Pan American Health Organization (PAHO) estimates that 7.7 million persons currently have T. cruzi infection, from the southern and southwestern United States to central Argentina and Chile. To date, the present treatments not solve the serious consequences of this disease.
This project is being performed in collaboration with the pharmaceutical company Laboratorio Silanes IDF, S.L., and the Facultad de Ciencias (Uruguay), which researh lines are:
Search of new lead compounds by means of artificial neuronal networks, optimization of the pharmacological properties and synthesis of new antichagasic drugs.
Design, optimization and synthesis of new cruzipain inhibitors by means of docking studies.
Chagas Disease

Chagas disease or American trypanosomiasis was discovered by the brazilian physician Carlos Chagas in 1909. The researcher completely described a new infectious disease indicating its pathogen, vector, clinical manifestations, epidemiology and some mammalian reservoir hosts.
Transmission of Chagas Disease

T. cruzi is transmitted to humans by haematophagous Triatomine insects (vinchuca in Uruguay, Argentina, Bolivia and Paraguay, barbeiro in Brazil, Pito in Colombia, chipo, chupança, chinchorro and also known as conenose bugs, kissing bugs, assassin bugs or triatomines) that are commonly found in poor areas with unhealthy housing conditions.
The current classification of the Triatominae recognizes 137 species in the order hemiptera, family Reduviidae, subfamily Triatominae grouped within 18 genera. Among them, Triatoma and Rhodinius and to a lesser extent Panstrongylus have greatest epide-miological importance as vectors of Trypanosoma cruzi. Other routes of transmission have also been described, as blood transfusion, congenital transmission, organ transplantation, ingestion of food contaminated with parasites and laboratory accidents.
The Parasite: Trypanosoma Cruzi
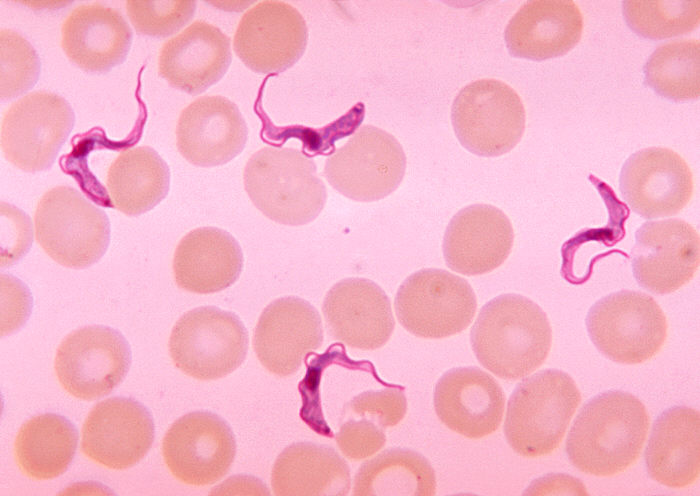
Trypanosoma cruzi belongs to the subkingdom Protozoa and to the subspecies A. stercoria, because the parasite develops its infectious stage in the insect’s digestive tract. They are flagellar organisms that have one nucleus and an organelle, the kinetoplast, that gives rise to one mitochondrion and mitochondrial DNA.
T. cruzi lives one stage of its life in the blood and/or tissues of vertebrate hosts and during other stages they live in the digestive tracts of invertebrate vectors as temporary hosts.
Trypanosoma cruzi
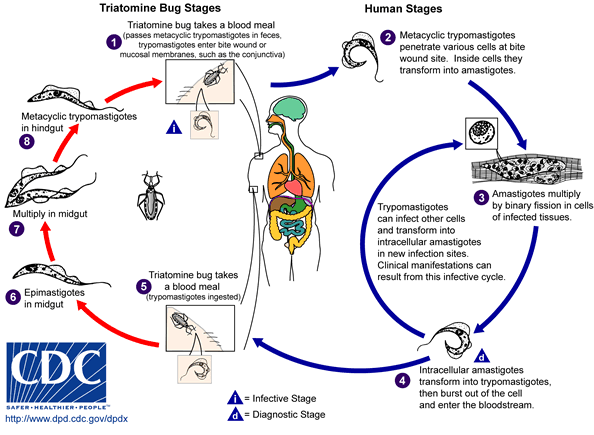
An infected triatomine insect vector takes a blood meal and releases trypomastigotes in its feces near the site of the bite wound. After metacyclic trypomastigotes pass through the skin, they briefly travel in the blood stream and then colonize muscle and neuron tissues, where they differentiate into intracellular amastigotes. The amastigotes are released into the circulation as bloodstream trypomastigotes. When triatomine insect feeds on blood from an infected animal or human, they ingest trypomastigotes. These trypomastigotes turn into epimastigotes in the vector’s midgut where the parasite proliferates and transforms to metacyclic trypomastigotes that will be released in the feces of the insect.
Drugs Against Chagas Disease
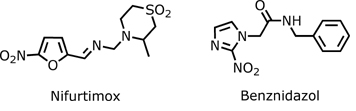
Currently, the only approved therapeutics for the treatment of Chagas disease are two nitroheterocyclic compounds, a nitrofuran (nifurtimox; Lampit) and a nitroimidazole (benznidazol; Rochagan) although in the United States these drugs are not approved by FDA (Food and Drug Administration) and are available only from Centers for Disease Control and Prevention (CDC) under investigational drug protocols. The production of nifurtimox was discontinued in 1997, first in Brazil, and then in Argentina, Chile and Uruguay. Currently, benznidazole is the only treatment commercialy available in most endemic countries. Two other alternative drugs for Chagas disease treatment are allopurinol and itraconazole that have been used in some selected cases and in certain conditions.
Anti-T. cruzi Drug Design
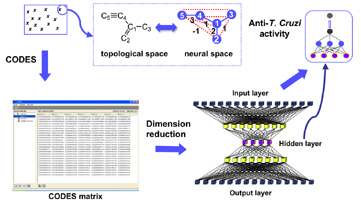
A supervised artificial neural network model has been developed for the accurate prediction of trypanocidal activity of any chemical compound. The artificial neural networks (ANNs) approach was used for the key steps of the development of a predictable model: descriptors generation using the CODES® program, dimension reduction strategy and model developing by means of supervised back propagation neural network.
The definition of the molecules was achieved from a non-supervised neural network using a new methodology, the CODES® program. (This program codifies each molecule into a set of numerical parameters taking into account exclusively the information of its chemical structure)
Cruzain Inhibitors
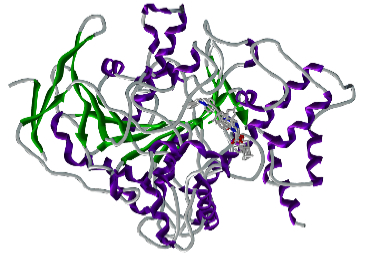
Cruzain (Cruzipain, GP57/51) is a member of the papain C1 family of cystein proteinases. The T. cruzi enzyme consists of a catalytic moiety with high identity to cathepsins S and L. and a particular C-terminal domain (C-T) which is absent in all other CPs of the C1 described families.
Irreversible inhibitors of cruzipain (peptidyl diazomethyl ketones, peptidyl fluoromethylketones, peptidyl vinyl sulphones) are able to block the differentiation steps in the parasite’s life cycle, and effectively kill the organism. Thus, cruzipain is an attractive potential target for the development of new drugs against Chagas disease.
Collaborations

Universidad de la República de Uruguay
Dr. Hugo Cerecetto and Dra. Mercedes González. Universidad de la República (URUGUAY).
Laboratorios Silanes
Drs. Jorge Paniagua and Jorge González (Mexico).
RIDIMEDCHAG (CYTED). Red de Investigación y Desarrollo para el estudio de la enfermedad de Chagas. (http://ridimedchag.fq.edu.uy/),
Dres. Hugo Cerecetto, Mercedes González, Dinorah Gambino y Graciel Mahler, de la Universidad de la República de Uruguay; Eder L. Romero (Universidad Nacional de Quilmes); Elizabeth Ferreira (Universidad de Sao Paulo); José Figueroa (Instituto Militar de Engenharia de Brasil); Luzineide Tinoco (Universidad Federal de Rio de Janeiro); Ximena Chiriboga (Universidad Central de Ecuador); Adela López de Cerain y Antonio Monge (Universidad de Navarra); Jorge Paniagua y Jorge González (Laboratorios Silanes IDF de México); Antonieta Rojas (Universidad Nacional de Asunción; Rosario Rojas (Universidad Cayetano Heredia de Perú); Jaime Charris (Venezuela).